Product name:Heparin sodium
CAS:9041-08-1
Molecular Formula:(C12H16NS2Na3)20
Specification:1G
Description:
Heparin Sodium Injection, USP is a sterile solution of heparin sodium derived from porcine intestinal mucosa, standardized for anticoagulant activity. It is to be administered by intravenous or deep subcutaneous routes. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
Heparin sodium is white or white powder, tasteless, has the ability to draw moisture, soluble in water, insoluble in ethanol, acetone and other organic solvents. It has strong negative charge in aqueous solution and can combine with some cation to form molecular complex. Aqueous solution is more stable at a pH of 7.
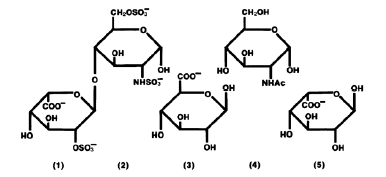
Heparin sodium is a natural anticoagulant substance containing sulfuric acid group. Heparin is the general name of a cluster of acidic mucopolysaccharide mixtures with different molecular weight sizes. It has linear chain molecules composed of hexosaccharide or ocosaccharide repeating units, with molecular weight ranging from 3000 to 30000 and average molecular weight around 15000.
Heparin sodium, together with other mucopolysaccharides, binds to proteins in tissues to form complexes. Therefore, the preparation process of heparin includes the extraction, dissociation and purification of heparin protein complexes.
Heparin contains sulfuric acid and carboxyl groups, which are strongly acidic and can react with cations to form salts. These cations include metal cations: Ca2+,Na+,K+, long-chain pyridine compounds of organic bases, such as cetyl pyridine chloride (CPC), strychnine, basic dye-cerurena, cationic surfactants (long-chain quaternary ammonium salts), such as cetyl trimethylammonium bromide; Cation exchange agent and positive protein such as fish essence 0 protein. The N- sulfate group in heparin structure is closely related to anticoagulation, and its anticoagulant activity decreases when it is destroyed. N- sulfuric acid groups are sensitive to acid hydrolysis and are quite stable under alkaline conditions. The free hydroxyl group in the heparin molecule is esterified, such as sulfurization, and its anticoagulant activity is also decreased, while acetylation does not affect its anticoagulant activity.

